THINK … TIME IS LIFE
How to discuss SUDEP with patients.

SUDEP RISK: a patient management perspective to achieving seizure freedom
“…the anxiety, that I could have a seizure at any time. I think that’s the biggest thing…”1
After being informed of an adverse event, people commonly overestimate the risk of that adverse event’s happening to them. Such overestimation unduly increases the anxiety related to an adverse event.2 The way healthcare professionals present data and interact with patients has a significant impact on risk perception. Overestimation can be lessened by presenting the risk as the probability of both having and not having the event and by using numbers in addition to words and frequencies rather than percentages to convey the risk.2
Patients are especially interested in factors that might reduce their risk even when a causal link between the factor and a reduction in risk has not been established. Knowledge of these risk factors might suggest behaviors that could modify the risk factors (e.g., improved therapy adherence), increase the person’s sense of control, and reduce the anxiety that comes from awareness of the risk.2
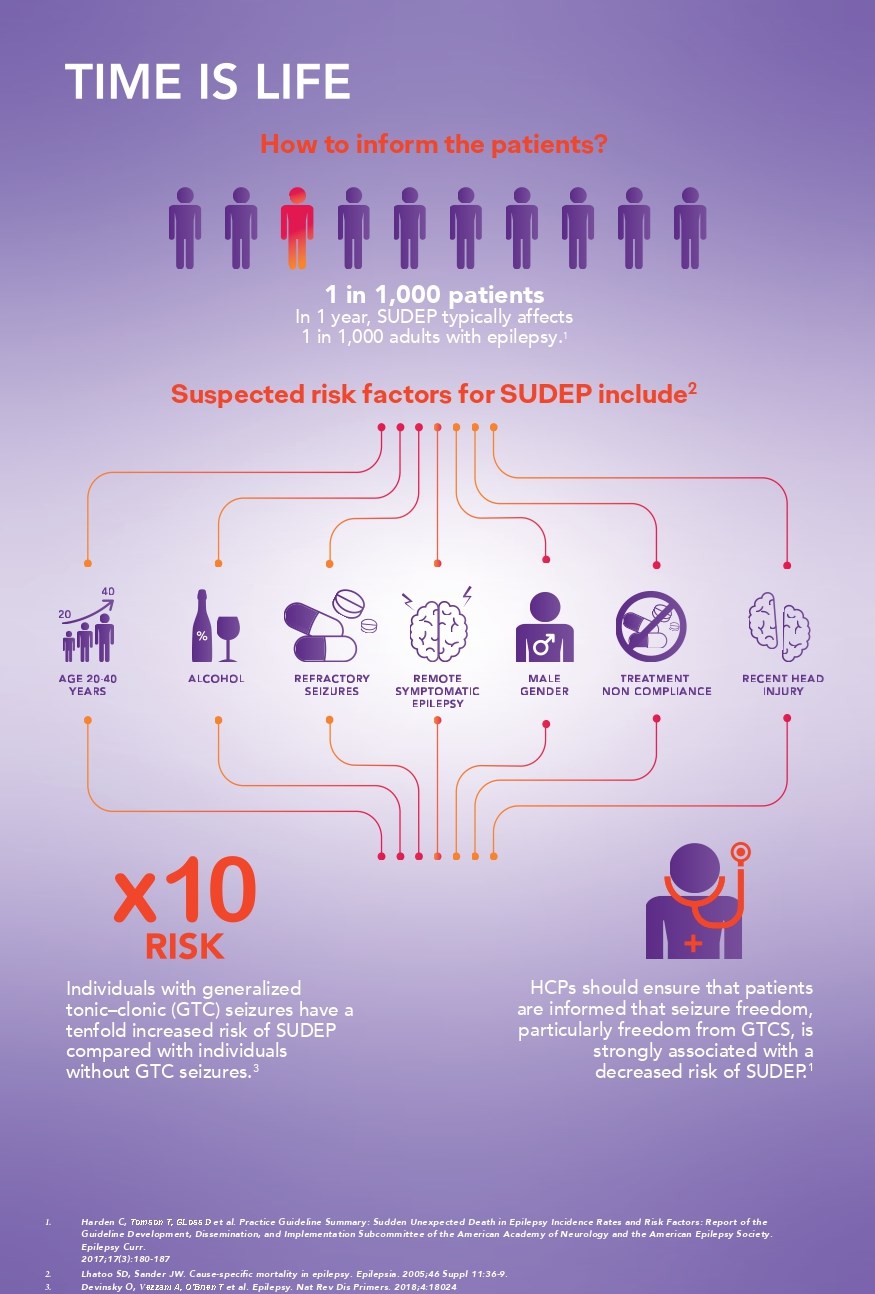
Effectively communicating SUDEP risk is an important aspect of epilepsy management.2,3
While the risk of SUDEP is relatively low2, it's important to remember that it remains a significant cause of epilepsy-related death in children and adults. 3This is particularly relevant in epilepsy surgery programs, where the incidence can be as high as 9.3/1000.4
Understanding SUDEP: Insights from Prof. Torbjörn Tomson
▼ Detta läkemedel är föremål för utökad övervakning.
Ontozry® (cenobamat) 12,5 mg odragerad tablett samt 25 mg, 50 mg, 100 mg, 150 mg och 200 mg filmdragerade tabletter. Rx F. ATC-kod: N03AX25 - antiepileptika, övriga antiepileptika. Indikation: Ontozry är indicerat som tilläggsbehandling av fokala anfall, med eller utan sekundär generalisering hos vuxna patienter med epilepsi, som inte kontrollerats tillräckligt trots tidigare behandling med minst två antiepileptika. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne, ärftligt kort QT-syndrom. Varningar: Patienter ska instrueras att uppsöka läkare om tecken på självmordstankar/självmordsbeteende uppstår, samt om tecken och symptom på läkemedelsreaktion med eosinofili och systemiska symtom (DRESS) inträffar. Innehåller laktos. Cenobamat kan minska exponeringen av substanser som metaboliseras via CYP3A4, CYP2B6 samt öka exponeringen av substanser som metaboliseras via CYP2C19. Cenobamat rekommenderas inte till fertila kvinnor som inte använder preventivmedel eller vid amning. MAH: Angelini Pharma S.p.A. Lokal kontakt: Angelini Pharma Nordics, nordic.medinfo@angelinipharma.com. Datum för senaste översyn av SPC: 2/2025. För pris och ytterligare information, se www.fass.se.
- Reeder S, Foster E, Vishwanath S et al. Experience of waiting for seizure freedom and perception of machine learning technologies to support treatment decision: A qualitative study in adults with recent onset epilepsy. Epilepsy Res. 2023;190:107096
- Harden C, Tomson T, Gloss D et al. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017 Apr 25;88(17):1674-80.
- Whitney R, Sharma S, Ramachandrannair R. Sudden unexpected death in epilepsy in children. Dev Med Child Neurol. 2023 Sep;65(9):1150-1156.
- Lhatoo SD, Sander JW. Cause-specific mortality in epilepsy. Epilepsia. 2005;46 Suppl 11:36-9.
MAT-SE-0005-P Mar 2025
 HarmoniaMentis Sverige
HarmoniaMentis Sverige

