THINK … EFFICACY, TOLERABILITY & LONG TERM
Retention rates and safety

"...It's just been a trial-and-error process since, which is very frustrating, mainly just because I don't really know where we go from here?"1
LONG-TERM EFFICACY, RETENTION AND SAFETY of CENOBAMATE
Epilepsy is one of the most common neurological disorders worldwide, with a prevalence estimated to be between 4 and 10 per 1000 people. Most of the people diagnosed with epilepsy are treated with antiepileptic drugs, and approximately 70% can become seizure-free once the most effective regimen is followed. However, non-adherence to medication is a prevalent and persistent healthcare problem and evidence indicates that poor adherence to medication is common among epilepsy-patients.2
Poor adherence to anti-epileptic drugs is associated with:2
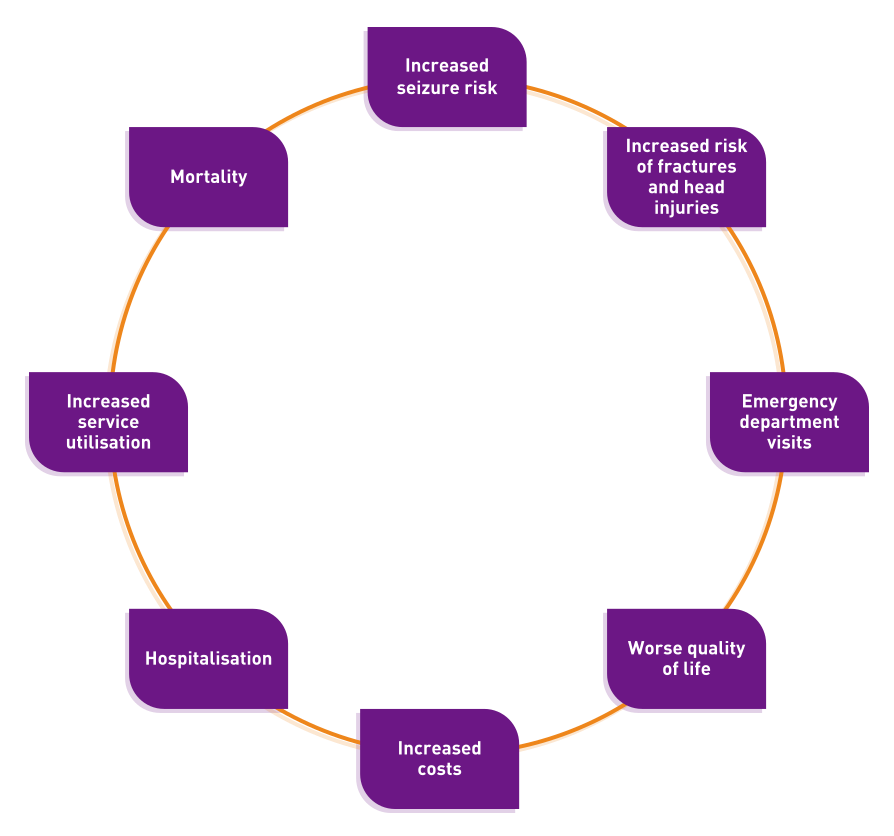
Reasons for poor adherence are varied but may include:3,4
| Beliefs about medications | Feeling depressed or anxious | Poor self-management |
| Side effects | Forgetfulness | Poor physician–patient relationship |
| Poor social support |
It is not easy to accurately measure adherence4 but the rate of non-adherence in adults with epilepsy is estimated to be between 29-66%.3,*
Retention rates for anti-seizure medications (ASMs) provide a composite measure of efficacy, tolerability, safety and adherence over a defined time period,5 and their use as an outcome measure is recommended by the European Medicines Agency (EMA) as a global indicator of a drug's clinical effectiveness.5
Data from retention studies may also provide benchmarks for interpreting and comparing clinical efficacy of ASMs, as well as evaluating long-term efficacy and tolerability beyond controlled clinical trials.5
High rates of retention on ONTOZRY® were observed during the clinical development programme5
Retention rates on ONTOZRY® appear to be comparable to or better than rates reported for ASMs in pre-marketing open-label extension studies conducted as part of respective clinical development programmes.5
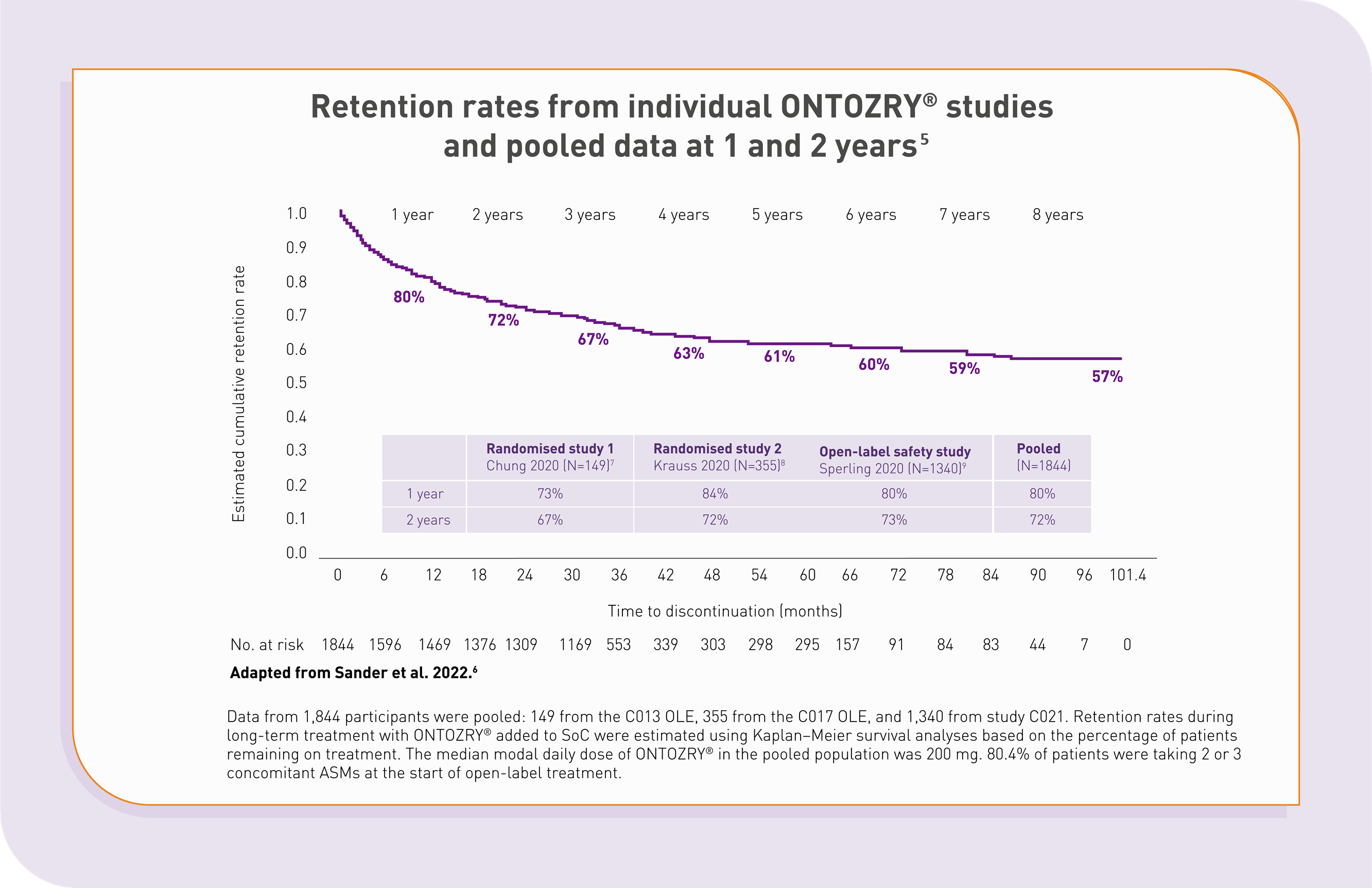
- High long-term retention rates with ONTOZRY® suggests sustained clinical efficacy and tolerability.5
- Concomitant anti-seizure medications did not impact retention.5
- These retention rates support the acceptability of the titration schedule and compliance with ONTOZRY6
Some aspects of safety
The most common TEAEs (treatment-emergent adverse event) across all three studies included (C013, C017 and C021) in analysis by Sander et al. were central nervous system related. Most TEAEs were mild or moderate in severity (75.8%)5
Cognitive and psychiatric side effects of ASMs can have importance in affecting treatment adherence and patient quality of life, so there is a need to understand the safety profile of treatments in terms of those adverse events.7
Recently published study by Krauss et al (2024), based on data from two phase 2 randomized, double-blind clinical trials, their open-label extensions, and a phase 3 long-term open-label safety study†, found that the occurrence of cognitive and psychiatric treatment-emergent adverse events (TEAEs) was low in patients receiving adjunctive cenobamate for up to 7 years, with fewer than 2% of patients discontinuing treatment due to these adverse events. This indicates a potentially good tolerability of cenobamate in regards of psychiatric and cognitive adverse events.7
Abbreviations:
AED = Anti-epileptic drug; ASM = anti-seizure medication; OLE = open-label extension; TEAE = treatment-emergent adverse event
*Data from a systematic review of adherence studies in adults with epilepsy who were prescribed AEDs.3
† C013, C013 OLE, C017, C017 OLE, C021
▼ Detta läkemedel är föremål för utökad övervakning.
Ontozry® (cenobamat) 12,5 mg odragerad tablett samt 25 mg, 50 mg, 100 mg, 150 mg och 200 mg filmdragerade tabletter. Rx F. ATC-kod: N03AX25 - antiepileptika, övriga antiepileptika. Indikation: Ontozry är indicerat som tilläggsbehandling av fokala anfall, med eller utan sekundär generalisering hos vuxna patienter med epilepsi, som inte kontrollerats tillräckligt trots tidigare behandling med minst två antiepileptika. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne, ärftligt kort QT-syndrom. Varningar: Patienter ska instrueras att uppsöka läkare om tecken på självmordstankar/självmordsbeteende uppstår, samt om tecken och symptom på läkemedelsreaktion med eosinofili och systemiska symtom (DRESS) inträffar. Innehåller laktos. Cenobamat kan minska exponeringen av substanser som metaboliseras via CYP3A4, CYP2B6 samt öka exponeringen av substanser som metaboliseras via CYP2C19. Cenobamat rekommenderas inte till fertila kvinnor som inte använder preventivmedel eller vid amning. MAH: Angelini Pharma S.p.A. Lokal kontakt: Angelini Pharma Nordics, nordic.medinfo@angelinipharma.com. Datum för senaste översyn av SPC: 2/2025. För pris och ytterligare information, se www.fass.se.
- Reeder S, Foster E, Vishwanath S et al. Experience of waiting for seizure freedom and perception of machine learning technologies to support treatment decision: A qualitative study in adults with recent onset epilepsy. Epilepsy Res. 2023;190:107096
- Al-Aqeel S, Gershuni O, Al-Sabhan J et al. Strategies for improving adherence to antiepileptic drug treatment in people with epilepsy (Review). Cochrane Database Syst Rev. 2020;lssue 10:CD008312.
- O’Rourke G, O’Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-8.
- Javor A, et al. Social cognition, behaviour and therapy adherence in frontal lobe epilepsy: a study combining neuroeconomic and neuropsychological methods. R Soc Open Sci. 2019;6:180850
- Sander JW, et al. Epilepsia. 2022;63(1):139-49
- Steinhoff BJ, Rosenfeld WE, Serratosa JM et al. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy—expert opinion. Epilepsy Behav 2021;123:108270
- Krauss GL, Chung SS, Ferrari L et al. Cognitive and psychiatric adverse events during adjunctive cenobamate treatment in phase 2 and phase 3 clinical studies. Epilepsy Behav. 2024 Feb;151:109605
MAT-DK-0021-P Apr 2025
 HarmoniaMentis Sverige
HarmoniaMentis Sverige

