THINK… EFFICACY
Ontozry (cenobamate) clinical trial program: Focus on C017

Ontozry (cenobamate) clinical trial program: Focus on C017
“The anxiety, that I could have a seizure at any time. I think that’s the biggest thing…” 1
A significant portion of epilepsy patients experience uncontrolled seizures despite available treatments, highlighting the urgent need for new, more effective therapies.2

Study C017, published in The Lancet Neurology, investigated the potential of adjunctive cenobamate to address this need. This pivotal trial demonstrated that cenobamate enabled dose-related reductions in focal seizure frequency among adults with uncontrolled focal seizures (1-3 existing ASMs). Significantly, 21% of patients taking the highest dose of cenobamate (400 mg/day) achieved seizure freedom.2
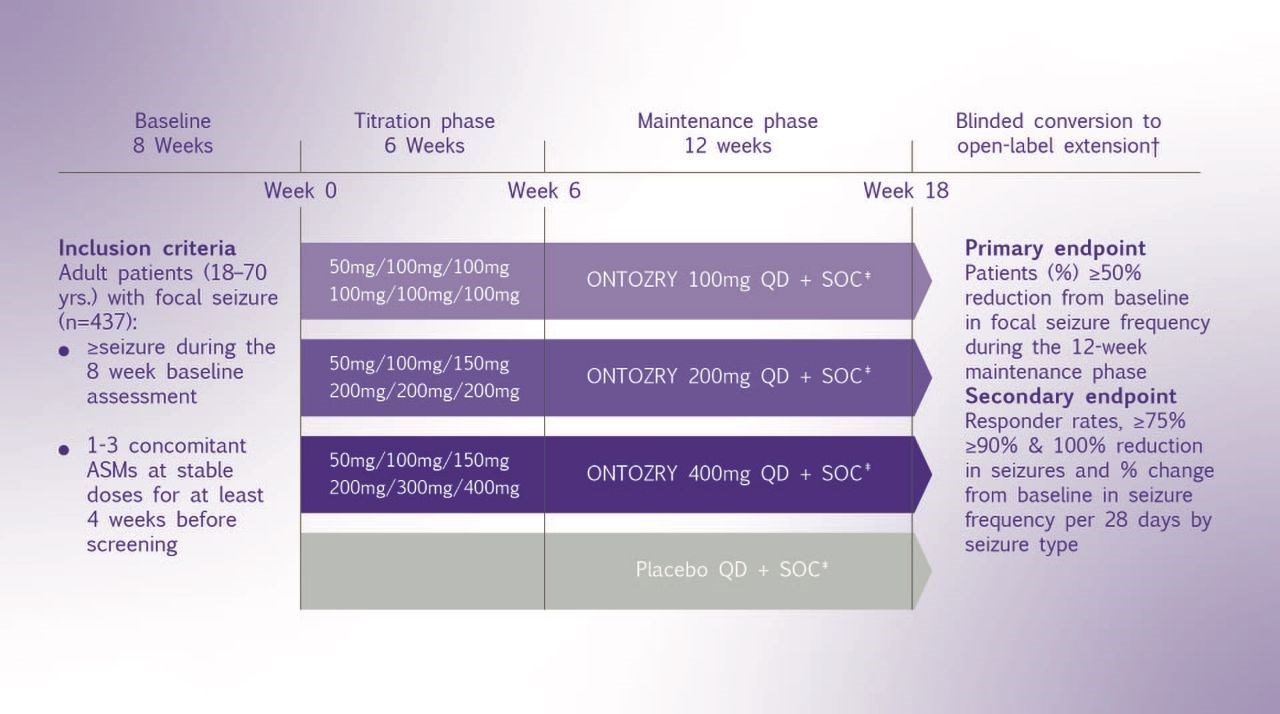
C017 Study Design:
The C017 trial was a multicenter, double-blind, randomized, placebo-controlled, dose-response study conducted at 107 epilepsy centers across 16 countries. The study enrolled 437 adult patients (aged 18-70 years) with a history of focal seizures who were experiencing inadequate seizure control despite ongoing treatment with 1-3 concomitant ASMs. Participants were randomly assigned (1:1:1:1) to receive adjunctive once-daily oral cenobamate at doses of 100 mg/day, 200 mg/day, or 400 mg/day, or placebo following an 8-week baseline assessment. The study included a 6-week titration phase, where the cenobamate dose was gradually increased, followed by a 12-week maintenance phase, where patients received their maximum attained dose of the study drug.2
Key Findings
-
Cenobamate demonstrated significant reductions in focal seizure frequency across all doses (100 mg, 200 mg, and 400 mg) compared to placebo. Furthermore, a dose-response relationship was observed, with higher cenobamate doses leading to greater seizure reduction. Measured only over a 12-week maintenance phase, significantly more patients treated with 200 mg and 400 mg of cenobamate achieved seizure freedom. Notably, cenobamate 400 mg was associated with a seizure-free rate of 21%, significantly higher than the 1% rate observed in the placebo group (p<0.0001). This finding is particularly encouraging, as achieving seizure freedom is a primary goal of epilepsy management.2
75% or more, 90% or more, and 100% seizure responder rates during the maintenance phase2

Adapted from fig 4 and text, ref. 2.
These seizure-free rates were further supported by a conservative analysis that considered only patients who completed the study without relying on dropouts as seizure-free cases. This analysis still demonstrated notable seizure freedom rates during the maintenance phase.2
-
Cenobamate was generally well-tolerated in this study. The most common adverse events were consistent with those observed with other ASMs and were primarily mild to moderate in severity.2
The C017 study provides robust evidence to support the efficacy and safety of cenobamate as an adjunctive therapy for adults with uncontrolled focal seizures. The dose-dependent reductions in seizure frequency, significant seizure-free rates and rapid onset of action suggest that cenobamate has the potential to be a valuable add-on therapy.2
Healthcare professionals are encouraged to stay informed about ongoing research and clinical trials evaluating the long-term effects and optimal use of cenobamate in the management of focal seizures.2
ASM = anti-seizure medication
▼ Detta läkemedel är föremål för utökad övervakning.
Ontozry® (cenobamat) 12,5 mg odragerad tablett samt 25 mg, 50 mg, 100 mg, 150 mg och 200 mg filmdragerade tabletter. Rx F. ATC-kod: N03AX25 - antiepileptika, övriga antiepileptika. Indikation: Ontozry är indicerat som tilläggsbehandling av fokala anfall, med eller utan sekundär generalisering hos vuxna patienter med epilepsi, som inte kontrollerats tillräckligt trots tidigare behandling med minst två antiepileptika. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne, ärftligt kort QT-syndrom. Varningar: Patienter ska instrueras att uppsöka läkare om tecken på självmordstankar/självmordsbeteende uppstår, samt om tecken och symptom på läkemedelsreaktion med eosinofili och systemiska symtom (DRESS) inträffar. Innehåller laktos. Cenobamat kan minska exponeringen av substanser som metaboliseras via CYP3A4, CYP2B6 samt öka exponeringen av substanser som metaboliseras via CYP2C19. Cenobamat rekommenderas inte till fertila kvinnor som inte använder preventivmedel eller vid amning. MAH: Angelini Pharma S.p.A. Lokal kontakt: Angelini Pharma Nordics, nordic.medinfo@angelinipharma.com. Datum för senaste översyn av SPC: 2/2025. För pris och ytterligare information, se www.fass.se.
- Reeder S, Foster E, Vishwanath S et al. Experience of waiting for seizure freedom and perception of machine learning technologies to support treatment decision: A qualitative study in adults with recent onset epilepsy. Epilepsy Res. 2023;190:107096
- Krauss GL, Klein P, Brandt C et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol 2020; 19(1): 38-48.
MAT-SE-0010-P Mar 2025
 HarmoniaMentis Sverige
HarmoniaMentis Sverige
